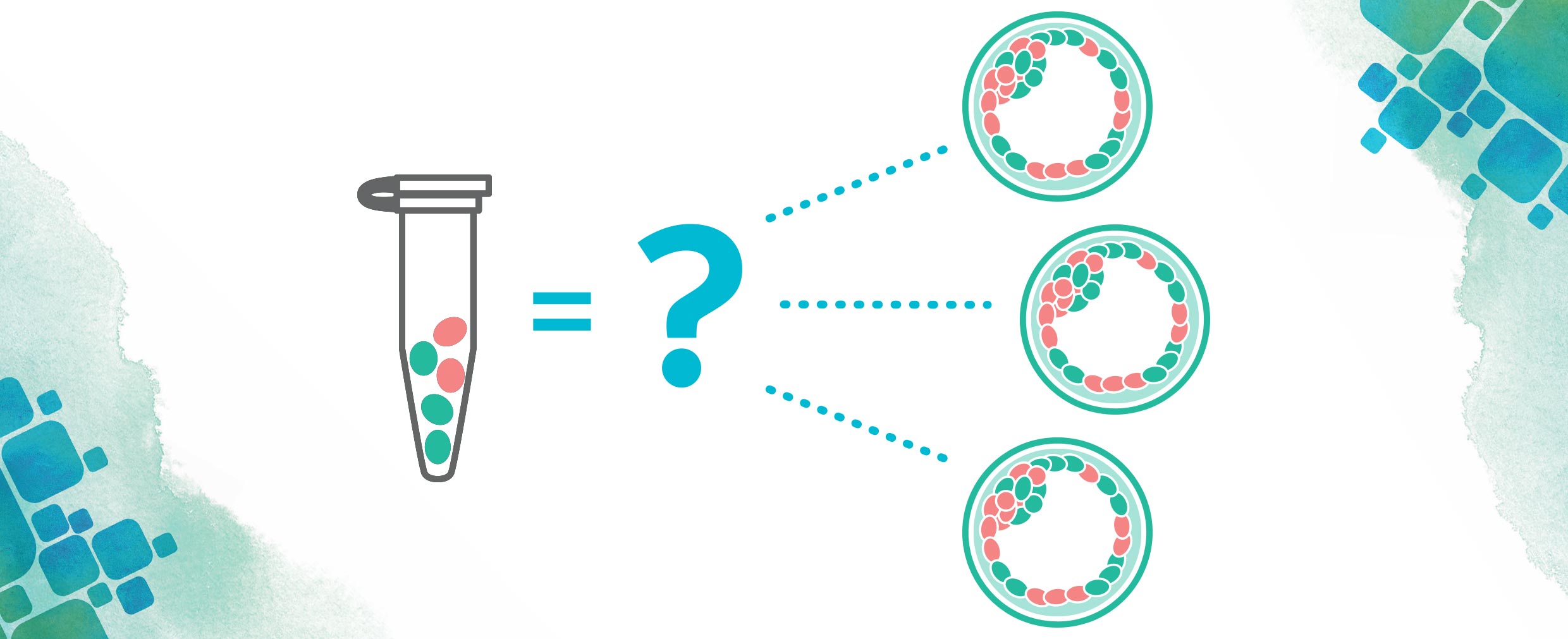
You have finally made the decision. You are going to pursue in vitro fertilization (IVF) to build your family. This may be the biggest decision you have made to date (believe me, I know. IVF veteran here!). Just when you think you have it all figured out: medication protocol, giving yourself an injection (or multiple injections), balancing your work schedule with all of the doctor’s appointments, someone (maybe your doctor, maybe a friend, maybe a fellow IVF warrior), asks you what genetic testing you are doing as part of your cycle. Um, what? Today, genetic testing is in all parts of medicine, and the fertility space is no exception.
While not every person wants or needs all available testing, here is a little information about the most common genetic tests you may encounter during your IVF journey.
Karyotype
A karyotype test is also referred to as a chromosome analysis; this is best completed before beginning an IVF cycle, if possible. Chromosomes are the structures that carry all of our genetic material. Most individuals have 46 chromosomes in each cell of their body. Chromosomes are inherited in pairs; one copy of each pair comes from the egg and one copy of each pair comes from the sperm. The first 22 pairs of chromosomes (the autosomes) are the same in males and females. The 23rd pair of chromosomes are the sex chromosomes. Individuals with two X chromosomes are chromosomally female. Individuals with one X and Y chromosome are chromosomally male. Some individuals have rearrangements in their chromosomes that may increase their chance to conceive an embryo with a chromosome abnormality1. One of the most common rearrangements that may be identified is a balanced translocation1.
This most often occurs when a piece of two different chromosomes break off and switch places. (reciprocal translocation)1.
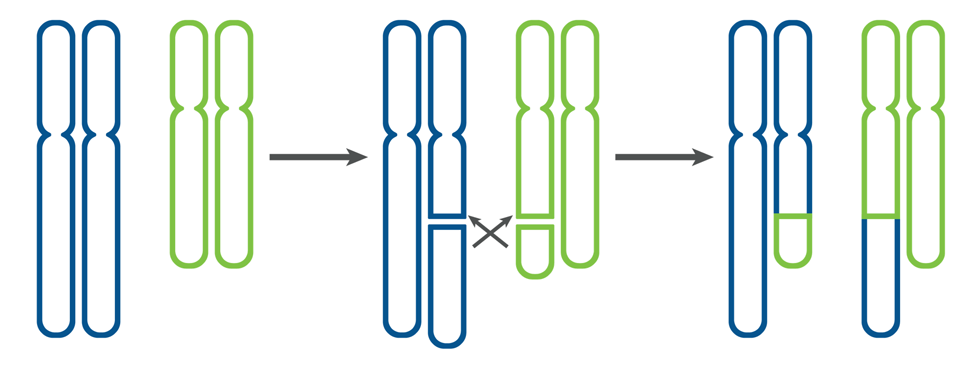
Figure 1
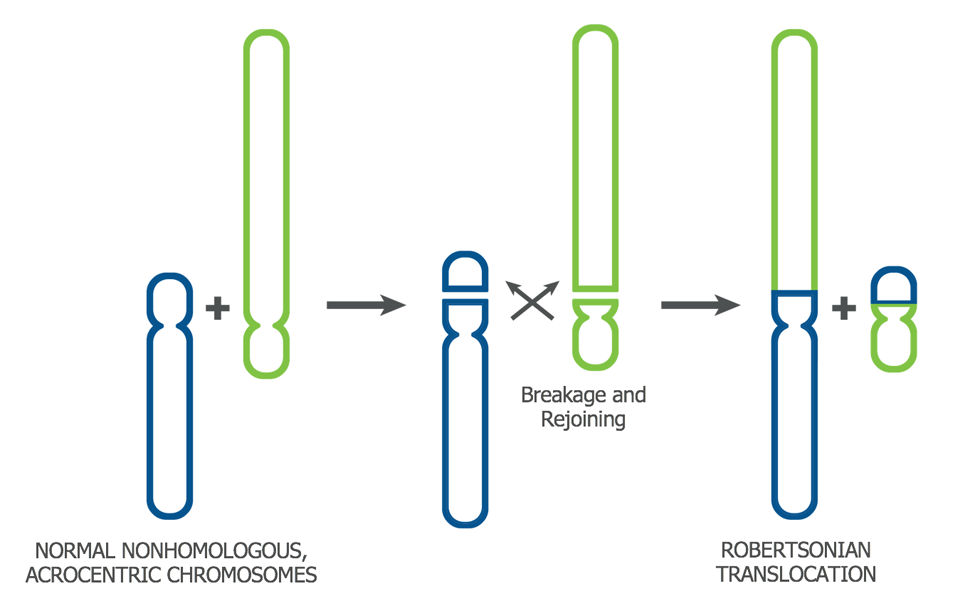
It may also occur when two whole chromosomes join end-to-end (Robertsonian translocation)1.
Preimplantation genetic testing for structural rearrangements (PGT-SR)
Identification of a translocation, or other chromosome rearrangement, before beginning an IVF cycle may allow the option of preimplantation genetic testing for structural rearrangements (PGT-SR). This testing evaluates embryo samples to determine if the correct amount of chromosome information is present, or if an unbalanced form of the rearrangement is present. Embryos with an incorrect amount of chromosome material often do not implant after transfer, or implant but miscarry1. Uncommonly, embryos with an incorrect number of chromosomes, including those with an unbalanced translocation, may implant and result in the birth of a baby with birth defects and/or intellectual disability1.
Carrier Screening
Carrier screening is also best completed before you start your IVF cycle, if possible. Carrier screening helps determine if you and your partner are at increased risk to have a child with certain inherited genetic disorders. Most disorders evaluated are inherited in an autosomal recessive manner, meaning if the person providing the egg and the person providing the sperm are both carriers, there is a 25% risk to have an affected child. Cystic fibrosis, sickle cell anemia, Tay-Sachs disease, and spinal muscular atrophy are common examples of autosomal recessive disorders, but there are many others. Carrier screening may also evaluate female patients for their carrier status of x-linked disorders. If a woman is a carrier of an x-linked disorder, in most cases, each male child has a 50% chance to be affected and each female child has a 50% chance to be a carrier. If a disorder is x-linked, males are often, but not always, more severely affected than females2. Fragile X syndrome and hemophilia type A and B are common examples of x-linked disorders, but there are many others.
Importantly, you cannot assume you are not a carrier of a genetic disorder if you have no medical issues and/or if you have no significant family history. Carriers of an autosomal recessive disease typically have no symptoms of the condition, and most do not have a family history of the disorder. Female carriers of an x-linked disorder may or may not have findings themselves and may or may not have a family history. This is why carrier screening is recommended for all individuals considering pregnancy, as well as for pregnant women who have not previously completed screening3.
If carrier screening finds you are a carrier of an x-linked disorder or finds you and your partner are carriers of the same autosomal recessive disorder, there are many testing options available to you, including the possibility of PGT-M (see below).
Preimplantation genetic testing for aneuploidy (PGT-A)
This test is completed after your IVF cycle, as it involves taking a small sample of cells, typically on day 5 or day 6, from the outer layer (the trophectoderm) of the embryo. These cells are then analyzed to determine the apparent chromosome complement. Current testing identifies whole chromosome gains, whole chromosome losses, and large missing or extra pieces of chromosomes. Embryo samples may be identified as aneuploid, meaning a chromosome abnormality is present in every cell tested, or as mosaic, meaning a chromosome abnormality is present in a portion of the cells tested. As all women have a risk of conceiving an embryo with an incorrect number of chromosomes, PGT-A may be considered by anyone completing an IVF cycle. However, it may be recommended for you if you have experienced multiple miscarriages, if you have had multiple unsuccessful transfers of untested embryos, or are over age 354.
Endometrial receptivity testing
This test evaluates the window of implantation (WOI); the time during which the endometrium (the lining of the uterus) is prepared to accept an embryo. For most women who are not undergoing fertility treatment, the WOI occurs 8-10 days after ovulation. For women who are completing a cycle of IVF, the WOI most often occurs five days after beginning progesterone supplementation. However, approximately 30%5,6 of women undergoing IVF or other assisted reproductive treatments (ART) have a WOI that occurs earlier or later than what is expected. Differences in the timing of the WOI may help explain why some couples experience multiple unsuccessful IVF cycles even when transferring embryos that have normal preimplantation genetic testing for aneuploidy (PGT-A) results. Endometrial receptivity testing requires an endometrial biopsy – removal of a small portion of the uterine lining at a specific time in the menstrual or hormone replacement cycle. Testing is typically able to determine if the WOI is occurring when expected (receptive), or if it is occurring earlier (post-receptive), or later (pre-receptive), than expected. These results may help your doctor decide the most appropriate time for your embryo transfer. For women who have experienced multiple unsuccessful transfers, studies have shown a significant improvement in pregnancy rates5,6 for those who have completed endometrial receptivity testing and have had their transfer date changed based on the result.
Navigating all of your genetic testing options may be overwhelming, but help is available. Your physician will help you determine what testing makes the most sense for your situation. You may also wish to speak with a genetic counselor as you are deciding what testing may be most appropriate. Whatever you decide, remember, the goal of genetic testing is to provide you actionable information while you are pursuing IVF. Good luck!
Sources:
1McKinlay Gardner RJ and Sutherland GR. Chromosome Abnormalities and Genetic Counseling, 3rd edition. Oxford, Oxford Press, 2004.
2U.S. National Library of Medicine. “What are the different ways in which a genetic condition can be inherited?”, 7 July 2020, https://ghr.nlm.nih.gov/primer/inheritance/inheritancepatterns.
3 American College of Obstetricians and Gynecologists. Carrier Screening in the Age of Genomic Medicine. Committee Opinion No. 690. American College of Obstetrics and Gynecologists, Obstetrics and Gynecology, 2017.
4Carvalho F, et al, ESHRE PGT Consortium good practice recommendations for the organisation of PGT, Human Reproduction Open, 2020.
5Ruiz-Alonso M, et al, The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure, Fertility and Sterility, 2013; 100:818–24.
6Tan J et al., The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers, Journal of Assisted Reproductive Genetics, 2018; 35(4):683-92.